
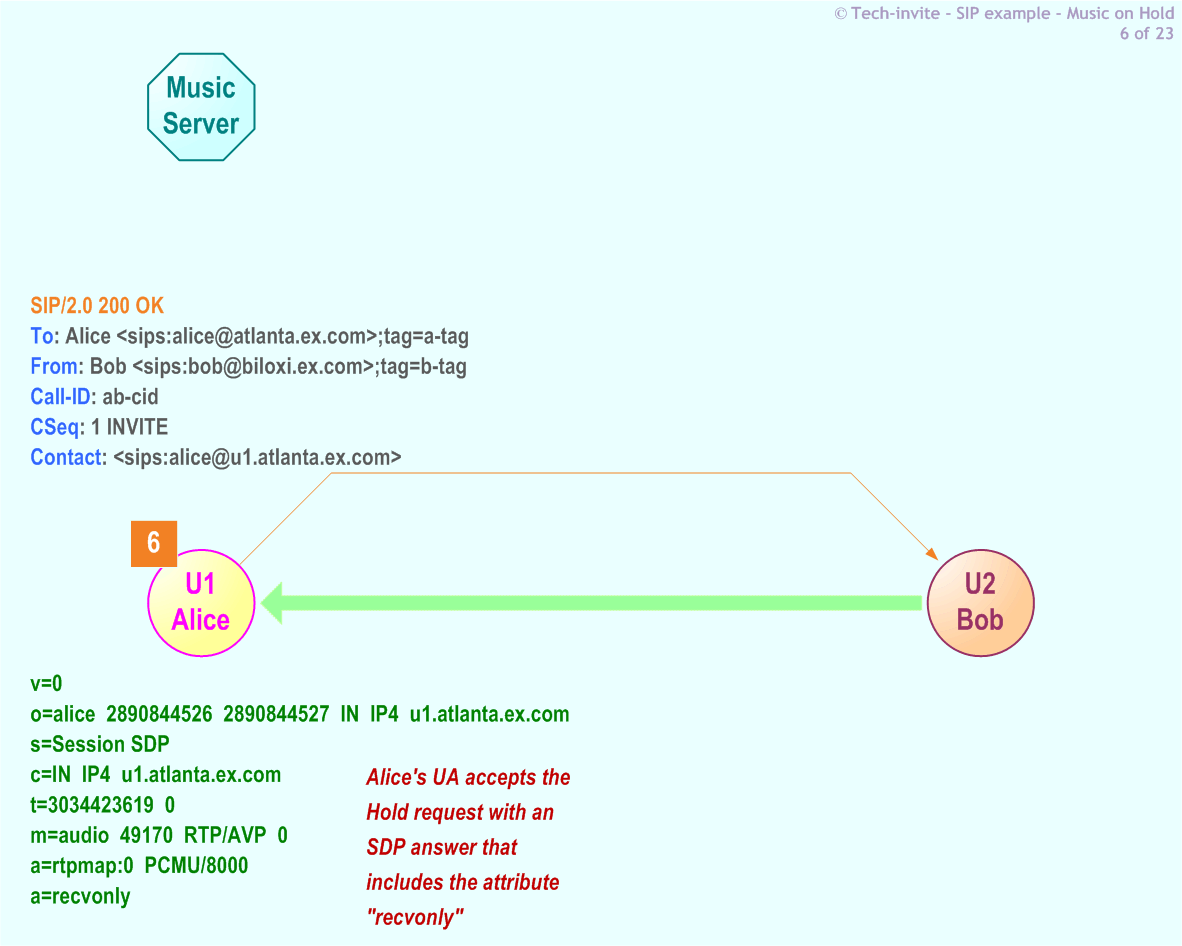
Integrity – Part 11 demands that electronic records and information generated by or managed for the organization must have a reasonable guarantee and verification of authenticity and reliability.The principles most relevant to Part 11 are Integrity, Compliance, Retention, and Protection: Doing so ensures data integrity while reducing fraud and security concerns.įrom a Records & Information Management (RIM) perspective, it is helpful to look at this regulation through the lens of ARMA International’s Generally Accepted Recordkeeping Principles® (GARP). Records and Information Management is considered as a companion discipline with enterprise data management, and shares many aspects of EDM / EIM.Įlectronic Record and Electronic Signature DefinitionsĪn Electronic Record is defined by Part 11 as “any combination of text, graphics, data, audio, pictorial, or other information representation in digital form that is created, modified, maintained, archived, retrieved, or distributed by a computer system.” This definition ensures that electronic records are the same as paper records.Īn Electronic Signature is defined by Part 11 as “computer data compilation of any symbol or series of symbols executed, adopted, or authorized by an individual to be the legally binding equivalent of the individual’s handwritten signature.” Part 11 contains requirements to ensure that electronic signatures have the legal standing equivalent to a person’s handwritten signature. Failure to comply with Part 11 can result in FDA citations and fines.
#SIP DEFINITION ELECTRONIC RECORDS SOFTWARE#
The regulation requires implementing controls such as internal audits, audit trails, system validations, electronic signature protocols, and documentation for software involved in processing the electronic data that FDA rules require to be maintained. Part 11 specifies how these FDA governed industries must handle electronic records and signatures and defines the criteria under which they are authentic, reliable, and equivalent to paper records.

Effective since 1997 and updated several times since, Part 11 applies to industries regulated by the FDA such as pharmaceuticals, biotechnology companies, contract research organizations (CROs), and medical device manufacturers. The United States Food and Drug Administration (FDA) established regulations for electronic records and signatures in Title 21 CFR Part 11 of the Code of Federal Regulations. Electronic records and their management have transformed how organizations collect, organize, and use a variety of information formats in any organization, not only pharmaceutical, biotechnology, and medical companies.


 0 kommentar(er)
0 kommentar(er)
